Basic Information
- Number of protons in the element define the element. Neutrons can change, electrons can change, but if protons change you have a different element.
- Fundamental elements (or elements with different number of protons in the nucleus) are found in the periodic table
- Number of protons is the atomic number (Z) of the element
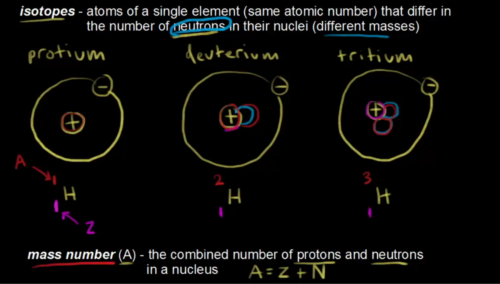
- Number of neutrons (N) + number of protons (Z) = atomic mass number (A)
- Isotopes are atoms of the same element which differ in number of neutrons (i.e. they differ in mass). [Image courtesy: https://www.youtube.com/watch?v=I-Or4bUAIfo]
- Same number of protons and electrons means it’s neutral. Neutral atom can become +ve or -ve if they depending on shedding or adding electrons.
- Most of all living things made out of carbon. ~1 million carbon atoms across width of the hair
What are ions?
- Carbon has 6 protons (and that is what it makes it carbon atom).
- A neutral carbon atom has 6 protons + 6 electrons. Usually when we use the term atom we refer to neutral atom.
- The way you get an ion is when you DON’T have the same number of protons and electrons.
- A carbon atom with 6 protons and 5 electrons is a positive ion (or Cation denoted by C
+ ) - A carbon atom with 6 protons and 7 electrons is a negative ion (or Anion denoted by C
– )
Electron configuration
- The electron diagrams that we see follow the bohr model which depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar to the structure of the Solar System.
- It is used to predict reactivity in elements which refers to how likely an element is to form a compound with another element.
- Valence electrons (the electrons on the last energy level) determine reactivity
- Rules we follow when laying out the model
- Max no. of electrons in a shell given by 2n2, e.g. 2, 8, 16, 32, 50…
- Max no. of electrons in the outermost shell is 8
- Electrons are not accommodated in a given shell, unless the inner shells are filled.
Ionic bonding
[Images Courtesy: https://www.youtube.com/watch?v=Qf07-8Jhhpc]
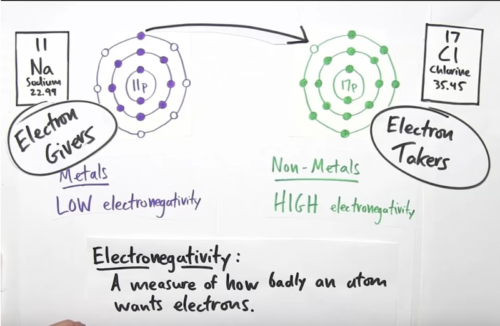
- Every atom wants a full valance shell!
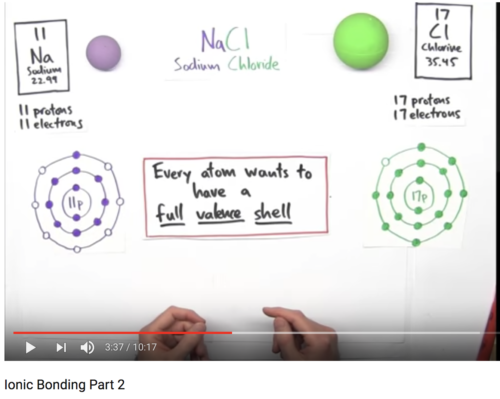
- Sodium gives its one (last) electron (from the valance shell) to chlorine. Now, both valance shells of sodium and chlorine are full.
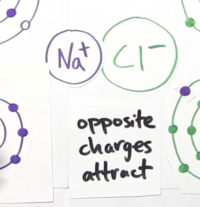
- Sodium now becomes a +ve charged particle while Chlorine becomes a -ve charged particle.

- Opposite charges attract, so both these atoms are attracted to each other forming sodium-chloride
- Ok, so one of the sodium electrons went to chlorine. Why didn’t the 7 electrons from Chlorine come to sodium?
Answer is electronegativity. A measure of how badly an atom wants electrons!